Abstract
Background: Taste disturbance, or dysgeusia, negatively affects quality of life and may lead to poorer nutritional status after high-dose melphalan and autologous stem cell transplantation (mel-AHCT) for patients with multiple myeloma (MM). Despite its frequency, dysgeusia has received limited prospective study. Here, we report the interim results of an on-going prospective clinical trial aimed at evaluating the feasibility of performing chemical gustometry, and quantifying the effects of dysgeusia on patient-reported symptom burden and nutritional status in MM patients undergoing mel-ASCT. We also sought to identify novel associations between mel serum and salivary pharmacokinetic (PK) levels and dysgeusia.
Methods: For this study, chemical gustometry was considered feasible for use as an endpoint in a future clinical trial if > 60% of the patients could complete > 60% of the evaluations. Chemical gustometry was performed according to the Henkin method (Henkin et al., Am J Otolaryngol 2013) at baseline (pre-AHCT) and on days -1, +7, +14, and +30 to test the 5 basic flavors: sweet (sucrose), sour (citric acid), salty (sodium chloride), bitter (quinine), and umami (monosodium glutamate). These data were used to calculate a total gustometry score (maximum value of 30). At the above time-points as well as day +3 and +10, caloric intake (kcal) was calculated using the USDA 5-Step Multiple-Pass Approach with 24-hour recall, and patient-reported symptom burden was assessed using the MD Anderson Symptom Inventory-Myeloma Module (MDASI-MM). Serum PK levels were drawn at 6 times points after mel infusion to calculate an area under the curve (AUC). Spearman rank-sum correlation was calculated for serum mel AUC and its association with total gustometry scores, caloric intake, and MDASI scores over time using a scaled 30-day AUC. A peak salivary mel level was measured at 75 minutes after infusion, and correlated with total gustometry scores, caloric intake, and MDASI scores.
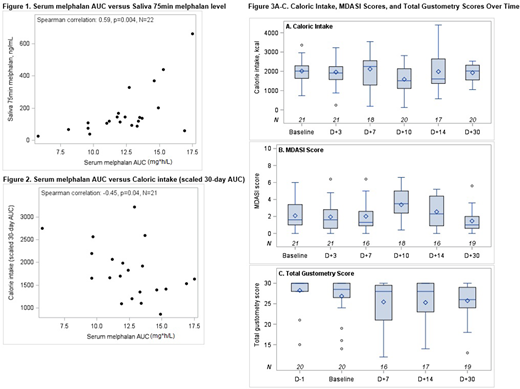
Results: To date, 22 patients with MM undergoing mel-AHCT have been enrolled and have follow-up data through day +30. Eighteen patients (82%) completed > 60% of all assessments. Median age was 62 (41-75), and 11 (50%) were female. Nineteen (86%) patients received mel 200 mg/m2 and 3 (14%) received mel 140 mg/m2. Median serum mel AUC was 12.6 mg*h/L (range 5.8-17.5), and median salivary mel was 119 ng/ml (range 25-662). Higher serum mel AUC correlated with higher salivary mel levels [Spearman correlation 0.59, p=0.004] as shown in Figure 1. Higher serum mel AUC was associated with poorer caloric intake from pre-ASCT to day +30 [Spearman correlation -0.45, p=0.04] as shown in Figure 2. Higher serum melphalan AUC was also associated with higher total MDASI scores (worse symptom burden) from pre-ASCT to day +30 [Spearman correlation 0.57, p=0.005], but was not significantly associated with total gustometry scores. A higher peak salivary mel level was associated poorer caloric intake from pre-ASCT to day +30 [Spearman correlation -0.49, p=0.02]. Among all patients, MDASI scores appeared highest on day +10, and total gustometry scores and caloric intake appeared lowest on days +7 and +10, respectively. At days +7, +14, and +30, 9 of 16 patients (56%), 8 of 17 patients (47%), and 9 of 19 (47%) had a drop of at least 1 point in gustometry score compared to baseline, respectively. Overall, gustometry scores appeared to improve by day +30, but often did not return to baseline (Figure 3A-C).
Conclusion: Chemical gustometry is feasible and comprehensively evaluates changes in taste function in MM patients after AHCT. The lowest gustometry scores were apparent on day +7 and often did not return to baseline by day +30, confirming the persistence of dysgeusia after AHCT. Moreover, higher serum and salivary mel levels are associated with poorer caloric intake and higher symptom burden scores within the first 30 days after AHCT. Preventing mel overexposure by targeted mel PK dosing may reduce dysgeusia, improve caloric intake, and limit symptom burden in MM patients after AHCT.
Shah:Janssen: Research Funding; Amgen: Research Funding. Peled:Seres Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.